After much speculation last week, US scientists have published a study in Nature in which they used CRISPR to edit human embryos, removing a mutation linked to a heritable heart condition.
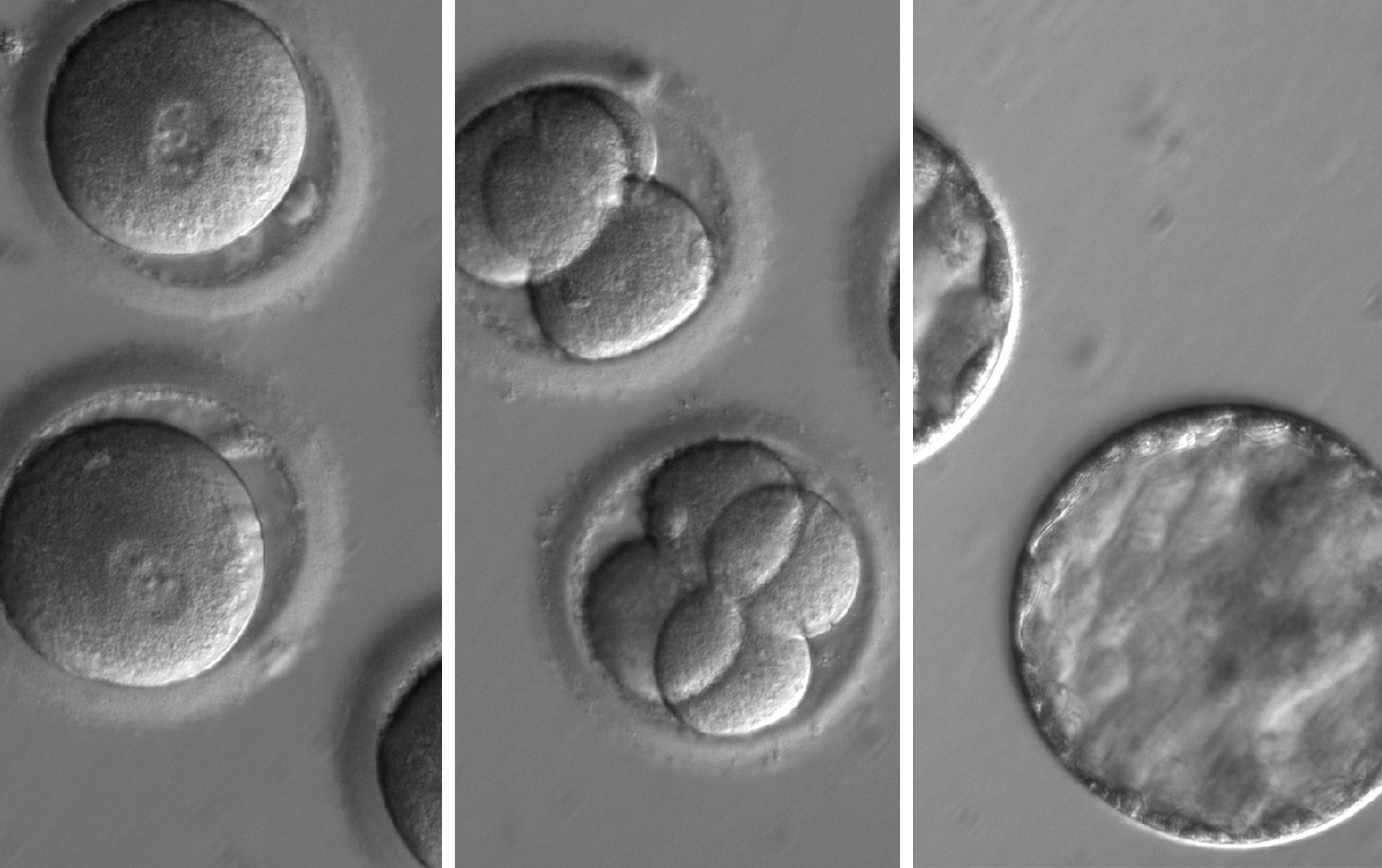
The researchers say more than 10,000 heritable disorders controlled by a single gene have been identified, and CRISPR-Cas9 gene editing provides a potential method to correct disease-causing mutations in human embryos.
The researchers were able to target the specific gene which causes the genetic heart disease hypertrophic cardiomyopathy very accurately and there was no evidence of off-target mutations.
More information about the research is available on scimex.org.
The SMC gathered expert reaction to the published study, please feel free to use these comments in your reporting.
Associate Professor Colin Gavaghan, Centre for Law and Policy in Emerging Technologies, University of Otago, comments:
“This is a promising beginning to what is going to have to be a prolonged investigation into the safety and efficacy of gene editing. It is a technique that offers a great deal in terms of treating devastating conditions, but there’s also considerable concern about potential risks.
“If it’s to be allowed here, there are also some legal hurdles to be overcome. In New Zealand at present, the legal status of this sort of research is somewhat in limbo. Human embryo research is only allowed on ‘non-viable’ embryos, but there are difficulties around determining precisely what that means.
“The UK and Australia both research on surplus embryos – those created during IVF treatment, but which have no prospect of ever being used to create a pregnancy. Proposals have been made to allow this in New Zealand, but for now, it couldn’t be done.
“In terms of using this technique for reproductive purposes, again the legal status isn’t exactly certain. The HART Act makes it illegal to ‘Implant into a human being a genetically modified gamete, human embryo’ – but there’s no definition within the Act of ‘genetically modified.’ Assuming it mirrors the definition in the HSNO Act, Parliament would need to make changes to the law before this technique could be used to create an embryo for reproductive purposes.
“In short, we’re quite some way – legally as much as technologically – from seeing gene editing in living humans, at least in New Zealand.”
Dr Jeanne Snelling, research fellow in bioethics and health law, University of Otago, comments:
What did the researchers do?
“The researchers used CRISPR-Cas9 to target and repair a particular gene mutation associated with hypertrophic cardiomyopathy in human embryos created by IVF. (Inheriting a single copy of such a mutation from a parent can cause heart failure and arrhythmias and, in some cases, may cause sudden death in otherwise healthy young individuals. Although CRISPR-Cas9 was used to ‘correct’ the mutation, none of the embryos were subsequently implanted.)
“Although this is not the first report of gene editing human embryos using CRISPR-Cas9 (the article cites three other reports since 2015) the researchers report advances in respect to two of the main technical hurdles associated with gene-editing in this particular case. The first is the problem of ‘mosaicism’ which occurs if some cells are edited and some are not, and the second problem involves ‘off-target’ effects, which may occur if the gene-editing components wrongly identify a similar sequence to that targeted and make unwanted DNA changes.
“In the study, donor eggs were fertilised using the sperm of men who carried one mutated copy of the gene, and one non-mutated version. CRISPR-Cas9 components were injected into the embryos when the embryos were at the single-cell (zygote) stage. The CRISPR-guided technology uses a RNA guide to identify the specific genomic target, which then triggers the Cas9 to cut the targeted DNA sequence causing a double-stranded break, which in turn activates a DNA-repair response. (Of interest is that most of these breaks were repaired efficiently using the healthy maternal gene copy as a template, rather than the synthetic DNA template injected with the CRISPR components.)
“Around two-thirds of the resulting embryos were found to contain two mutation-free copies of the targeted gene. Significantly, no off-target edits were observed in the study, and the embryos developed similarly to the control embryos. The level of accuracy achieved in the study is striking, although the authors note it may not be replicated in other targeted gene sequences and further research is necessary.
Possible implications of CRISPR
“Currently, individuals who carry a disease-causing mutation but wish to avoid transmitting it to their offspring may elect to undergo preimplantation genetic diagnosis (PGD), a process that involves undertaking a cycle of IVF and biopsying the resulting embryos to test and select a mutation-free embryo.
“If further research and subsequent clinical trials establish that CRISPR technology is safe and accurate in the future, it could be used in conjunction with PGD to correct mutations. However, given the nature of the stakes involved and the current preliminary stage of the research, that scenario not imminent.
“As well as issues regarding safety, gene-editing creates heritable changes, which may be passed on to subsequent generations and which create additional ethical, social and legal issues. The issues raised by gene editing are currently being considered by a multidisciplinary panel established by the Royal Society Te Apārangi to consider the implications of gene-editing technologies for NZ. The panel is co-chaired by Dr David Penman and Professor Barry Scott.
Can this kind of research be done in NZ?
“Human reproductive research is currently governed by the Human Assisted Reproductive Technology (HART) Act 2004 and guidelines made under the Act. The Act requires that human reproductive research must be approved by the Ethics Committee on Assisted Reproductive Technology. (Guidelines for Research on Gametes and Non-viable Embryos, 2005). Research involving genetic modification of human gametes and embryos also comes within the regime established by the Hazardous Substances and New Organisms Act 1996.”
Can gene-edited embryos be used in IVF in NZ?
“The HART Act 2004 prohibits the implantation of a genetically modified embryo into a woman.”
Our colleagues at the UK, German and Australian Science Media Centres also gathered expert reaction to the published study.
Dr Nathan Palpant, Group Leader, Genomics of Development and Disease Division, Co-Director, Queensland Facility for Advanced Genome Editing, Institute for Molecular Bioscience, University of Queensland, comments:
“Acquired genetic diseases remain a significant cause of morbidity and mortality worldwide and provide significant rationale for in vitro fertilization and genetic testing of embryos.
“In recent years, the development of genome editing technologies such as CRISPR-Cas9 has provided a powerful means to modify DNA. While researchers use this technology routinely for experiment work, the application of this approach in human embryos is a significant advance with implications for manipulating the genome of future generations.
“The publication by Ma et al in Nature provides important new insights into the use of CRISPR-Cas9 for gene correction of disease-causing mutations. The findings reveal new mechanisms by which DNA repair occurs in the embryo and evidence for specificity of the genetic modification. These findings add to a growing field of studies showing the power of CRISPR-editing as a therapeutic for acquired and inherited diseases.
“The ethical challenges of defining the boundaries for genome editing in this field will require careful consideration as we increasingly gain the capacity to manipulate the human genome.”
Professor Nigel McMillan, cancer biologist, Griffith University’s Menzies Health Institute Queensland, and President of The Australasian Virology Society, comments:
“This is a big leap forward and quite a remarkable study which has really addressed the issues previously seen with gene editing whereby off-target (unwanted) effects were always a by-product of the technology. Previously the problems with a genetic mistake were fixed, but not without also causing unwanted changes to other genes.
“This is a real breakthrough in that the researchers have shown they can repair their target gene within the embryo, which causes heart disease, without producing any off-target effects.
“It is obvious that CRISPR technology has the ability to alleviate the suffering that is caused with many genetic conditions, e.g. cystic fibrosis, however it could also allow for the manipulation of human traits such as height, eye colour etc., as well as many other applications such as crop and animal production.
“This technology currently remains in the testing stage and these latest developments raise further ethical questions for society to consider, much like IVF treatment was up for debate forty or fifty years ago.”
Prof Peter Braude, Emeritus Professor of Obstetrics and Gynaecology, King’s College London, comments:
“This remarkable paper demonstrates just how rapidly the field of genome editing has progressed since the CRISPR-Cas9 system was voted science breakthrough of the year in 2015. The substantial author list also shows the very best of international collaboration (USA, China and South Korea) in order to bring high-end genetic and cellular technology to bear on a technically difficult and controversial area of science and medicine.
“Whilst there are still some important potential hazards such as mosaicism and off-target effects, substantial progress has been made here on understanding how they might happen and be ameliorated.
“Preimplantation genetic diagnosis (PGD) with embryo selection is still the only current practical option for couples to prevent transmission of genetic disorders to their offspring, but this paper presents a possible future alternative especially in dominant disorders (like Marfan syndrome, Huntington disease or Hypertrophic Cardiomyopathy as in this paper), as the editing correction seems more reliable when there is one normal gene present as is usually the case in such inheritance. Corrective editing could reduce the proportion of embryos discarded as being not suitable for transfer after PGD.
“Although use of this method clinically would not be allowed under current legislation in this country [the UK], with this paper the possibility of germline genome editing has moved from future fantasy to the world of possibility, and the debate about its use, outside of fears about the safety of the technology, needs to run to catch up.”
Prof Robin Lovell-Badge, Group Leader, The Francis Crick Institute, comments:
“The headlines from the ‘scoop’ of this paper last week were clearly wrong. The paper is not about designer babies. It is aimed at preventing the inheritance of dominant mutations, in this case one that confers a severe risk of early onset cardiac disease that is a common cause of sudden death in young athletes. They show that the methods may work, although not in the way that was anticipated.
“Sperm was obtained from a man heterozygous for the mutation in the MYBPC3 gene, which were used to fertilise normal eggs donated for research. By introducing the genome editing components along with the sperm, the mutation was corrected at high efficiencies in the 50% of embryos that should have inherited the mutation (much higher than the rate seen in cells in culture), and importantly, they managed to avoid mosaicism (in all but one embryo). However, rather than repairing the mutation with a DNA template introduced along with the Cas9 and guide RNAs, the repair mechanism used the maternal (the egg’s) copy of the gene. This was surprising, as it would be expected that the many copies of the DNA template would be used in preference to the single maternal copy. This is not simply ‘mother knows best’, but it suggests that a novel mechanism of DNA repair operates in this very early stage of human embryo development.
“There is still much to be done to establish the safety of the methods, therefore they should not be adopted clinically. However, this mechanism of genome editing could, in theory, be harnessed to correct mutations coming from a father; as in the case described, to increase the chances of obtaining an embryo that does not carry a dominant disease-causing version of a gene. Alternatively, if the father was homozygous for such a mutation, it would allow him to have a genetically-related unaffected child, which would otherwise not be possible. However, it seems to be a one-way repair process – it would probably not work if the mother were carrying the mutant gene (indeed, it might lead to embryos homozygous for the mutation). This will require new methods to permit efficient DNA template-directed repair. The mechanism revealed in this paper would similarly not permit more sophisticated genetic alterations. The possibility of producing designer babies, which is unjustified in any case, is now even further away.”
Prof. James Adjaye, Chair of Stem Cell Research and Regenerative Medicine, Heinrich Heine University, Germany, comments:
“The design of this study is well thought of and executed, especially regarding the comparative use of patient-derived induced pluripotent stem cells (iPSCs) and the derived embryos after in vitro fertilization. A major and unexpected observation was that it seems that the DNA-repair mechanism(s) in the early embryo differs from that operative in the iPS cells and maybe even somatic / adult cells.
“This is an important finding for basic research and also a necessary pre-requisite for correcting mutation at the oocyte or preimplantation embryo level. However, the results are currently based on one single patient as donor, so additional replicates/ donors are needed to statistically validate this finding.
“In earlier studies on CRISPR-Cas9 editing in human preimplantation embryos the authors added the gene-editing components after fertilization in contrast to the current study. The low levels of mosaicism observed in this study can be attributed to the fact that gene editing occurred before the first cell division took place. Interestingly the gene-corrected preimplantation embryos developed in a similar manner to the control embryos, with 50% developing to the blastocyst stage which is the stage where the embryos have the potential to develop further into the fetus and placenta.
“Ma et al. did not observe off target effects and this they suggest might be due to the fact that they—unlike other previous investigators—used purified recombinant Cas9 protein instead of plasmid DNA, which might have enhanced the specificity due to a more controlled level of Cas9 enzyme activity.
“All said and done, more experiments are needed to address these and other yet to emerge questions before CRISPR-Cas9 can be routinely implemented in therapy.
“Pre-implantation Genetic Diagnosis (PGD) still remains the favoured and standard approach in preventing the transmission of deleterious genetically inherited diseases to offspring.”