Research published today in the journal Nature has found that stem cells from mice have an unexpected ability to spontaneously organise themselves into a complex structure that resembles the developing embryonic eye.
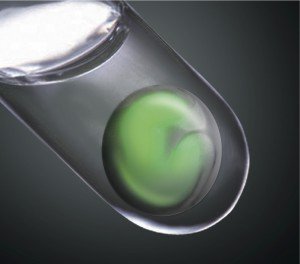 This ground-breaking research shows that embryonic stem cells, under the right conditions, can spontaneously transform into intricate tissues. The Japanese scientists behind the study hope the findings will aid the development of stem cell-derived transplants for retinal repair.
This ground-breaking research shows that embryonic stem cells, under the right conditions, can spontaneously transform into intricate tissues. The Japanese scientists behind the study hope the findings will aid the development of stem cell-derived transplants for retinal repair.
The following comments from the authors and independent researchers were collected by our colleagues at the Australian Science Media Centre, in collaboration with the Science Media Centre of Japan with support from the Australia-Japan Foundation.
Feel free to use these quotes in your stories. Images and a video are available upon request. For more information, or to talk to local experts, contact the Science Media Centre on (04) 499 5476, smc@sciencemediacentre.co.nz
———–
 Dr Yoshiki Sasai is Group Director of the Laboratory for Neurogenesis and Organogenesis at Japan’s RIKEN Center for Developmental Biology in Kobe. He is an author of the Nature paper.
Dr Yoshiki Sasai is Group Director of the Laboratory for Neurogenesis and Organogenesis at Japan’s RIKEN Center for Developmental Biology in Kobe. He is an author of the Nature paper.
“What we’ve been able to do in this study is resolve a nearly century-old problem in embryology, by showing that retinal precursors have the inherent ability to give rise to the complex structure of the optic cup.
It’s exciting to think that we are now well on the way to becoming able to generate not only differentiated cell types, but organized tissues from ES and iPS cells, which may open new avenues toward applications in regenerative medicine.”
———–
Dr Stephanie Watson works in the Corneal Unit at the Prince of Wales, Sydney Children’s and Sydney Eye Hospitals. She is also an Associate of the Save Sight Institute at the University of Sydney and a Conjoint Senior Lecturer at the University of New South Wales
“This paper is a significant advance in ocular stem cell research as rather than generating a colony of cells of a single cell type Eiraku et al report the in vitro morphogenesis of retinal tissue. The retina is a highly complex structure composed of numerous interconnected cell types. Success in retinal stem cell therapeutics has to date been limited by the inability to recreate retinal tissue.
This study has shown that, at least in vitro, retinal morphogenesis [embryonic development of the eye] is a dynamic process that is internally and locally controlled. Such that as well as providing retinal tissue, further research may be able to identify factors and internal mechanisms that could be used in retinal repair.”
———–
Professor Bernie Tuch is Director of the NSW Stem Cell Network
“I am quite excited about the broad aspects of the research described in this paper.
Cell therapies are being developed for treatment of disorders such as diabetes, liver disease and Parkinson’s Disease, conditions where individual cells that have been destroyed might be replaced with surrogate cells of a similar type, that are developed from stem cells.
Replacing organs, for example, an eye or a kidney, is a much more difficult scenario because there are multiple different cell types, that need to integrate correctly with one another in a 3D environment. Our knowledge of what different cell types interact during development and when these interactions occur is only beginning to be understood.
This manuscript describes the creation of the basics of a very primitive eye (“optic cup”) in the laboratory, with the series of interactions between the various developing cell types that make up the eye occurring spontaneously once the initial steps were induced. Initiating the process required aggregates of mouse embryonic stem cells growing in a specific type of culture medium to be placed on a supportive matrix of proteins in the laboratory.
The message from this manuscript is that if appropriate stimuli are provided to embryonic stem cells in the laboratory, organ development can begin to be induced. Whilst what was described relates to the eye, it is theoretically possible the same outcomes might be achieved with other organs using different stimuli. Whether the primitive optic cup that was formed can mature and differentiate all the cell components of an adult eye is unknown, but this manuscript raises the hope that this might be possible one day.”
———–
Professor Andrew Elefanty is from Monash Immunology and Stem Cell Laboratories, Monash University, Victoria
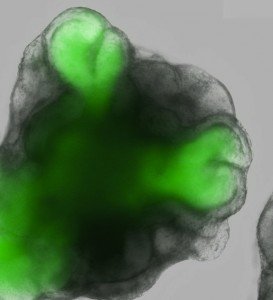
“In this study published in the premier journal, Nature, scientists from the RIKEN Center for Developmental Biology in Kobe, Japan, have taken mouse ESCs and cultured them to reproduce the early stages of eye development. They showed that culturing the cells (which were modified to glow green at the first stages of eye formation) in a specific fashion was necessary to allow the formation of a cup-like structure mimicking that seen in the development of the eye in a developing mouse. Using sophisticated imaging analyses they confirmed that these structures indeed were very similar to the early developmental stages of the eyes in embryos. The authors demonstrated that their cultures contained several different cell types, interactions between which were necessary for the eye-like structures to develop.
This work is important for several reasons. Firstly, it shows that it is possible to start to form a structure as complicated as the eye in the laboratory. Secondly, it highlights the value of sophisticated gene modification and imaging technologies in identifying the correct conditions to make different types of cells in the laboratory. Thirdly, it provides a starting point for the formation of the complex nerve cell layers that are needed to transmit visual signals from the outside world to the brain. The hope is that the culture systems that these scientists have developed will lead to discoveries that will eventually enable the growth of sheets of human retinal cells both for the study of eye diseases and eventually for the growth of new cells to treat patients with blindness. This last wish, however, is likely to still be some way off.
The development of organs and tissues from stem cells to replace those lost through abnormalities of development, accident or disease represents one of the major challenges in regenerative medicine. The formation of tissues like the heart, liver, lungs, pancreas and nervous system is complex, relying on physical interactions between different types of cells plus various growth promoting molecules, all coming together at the right place and at the right time during normal development.
Pluripotent stem cells (PSCs, the term used to encompass embryonic stem cells (ESCs) cultured in the laboratory from early embryos and induced pluripotent stem cells (iPSCs) generated by ‘reprogramming’ adult cells with extra genes and/or chemicals) can make all the different types of cells in the body. However, the task of mimicking the organization and combinations of cells observed in the embryo that are needed to make a complex organ has seemed to a huge task.
Huge, perhaps, but not hopeless. Studies in many laboratories have shown that some basic elements of normal tissue development are preserved when PSCs are differentiated in the culture dish. Firstly, we know that these cells respond to the same growth promoting molecules that are normally present in the embryo in the same way – an observation that enables us to regulate to some extent the type of cells that develop, such that we can direct cells towards cell types like blood, heart, pancreas and nerve tissues. Secondly, we know that some complex structures can develop in the culture dish. Most obvious is the formation of beating sheets of muscle cells that indicate the development of heart structures. Scientists have also reported the formation of structures reminiscent of developing pancreas, intestine and lung.”
———–
Article: Self-organizing optic-cup morphogenesis in three-dimensional culture, Eiraku et al. doi:10.1038/nature09941 (2011) VOL.472 NO.7341, 7 April 2011 (Nature)
Image credits: M. Eiraku and Y. Sasai at RIKEN Center for Developmental Biology
Images and a video are available upon request. For more information, or to talk to local experts, contact the Science Media Centre on (04) 499 5476, smc@sciencemediacentre.co.nz