New drug legislation has been used a second time to quickly ban emerging psychoactive substances thought to be on the brink of being marketed as “legal highs”.
 From today, three new synthetic cannabinoids have been classified as ‘temporary class drugs’ – meaning that for one year they are legally considered a Class C drug, (equivalent to cannabis) with the exception that personal possession is not criminalised.
From today, three new synthetic cannabinoids have been classified as ‘temporary class drugs’ – meaning that for one year they are legally considered a Class C drug, (equivalent to cannabis) with the exception that personal possession is not criminalised.
The substances, which are prohibited as of today, are:
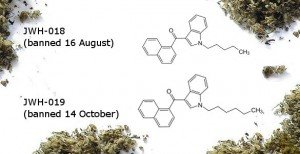
JWH-019
1-hexyl-3-(1-naphthalen-1-oyl)indole
JWH-200
(1-(2-(morpholin-4-yl)ethyl)indol-3-yl)-naphthalen-1-ylmethanone
AM-1220
(1-((1-methylpiperidin-2-yl)methyl)-1H-indol-3-yl)(naphathen-1-yl)methanone
All three chemicals are cannabinoids, having an effect on the brain similar to THC (the active substance in cannabis).
The classification was gazetted on October 7, to take effect immediately (in line with the Misuse of Drugs Act). Associate Minster of Health Peter Dunne foreshadowed the change in a press release earlier this month.
The Temporary Drug Class Notice legislation was introduced and first used in August to ban 16 synthetic cannabinoids which were found in legal smoking blends such as Kronic.
JWH-019 is one carbon atom different from JWH-018, the most common synthetic cannabinoid found in synthetic cannabis products before it was banned in August. The 43 products tested by ESR before August this year included 39 which contained JWH-018.
The Government was tipped off by the Customs Department to the import of these substances, though it is not yet known these specific chemicals are the active ingredients in new smoking blends being marketed (such as Amsterdam Cafe).
JWH-200 and AM-1220 were first synthesised as experimental chemicals to be used in research over 20 years ago but have been recently been noted as ingredients in ‘legal high’ products overseas.
The Science Media Centre gathered the following expert commentary on synthetic cannabinoids and current legislation.
Keith Bedford, General Manager Forensic, Institute of Environmental Science and Research Limited (ESR), comments:
“The three additional substances listed in the new Temporary Class Drug Notice (TCDN) are very similar to the other synthetic cannabinoid-like substances listed earlier. There are very likely to be others that appear in due course because a significant number of analogues of these substances have been reported in the scientific literature and/or in overseas reports.
“It was accepted that additional substances would need to be listed via a Temporary Class Drug Notice as they came to the attention of authorities and the TCDN mechanism provides for new substances to be added very quickly.
“Very limited information is available in the scientific literature on the toxicity and effects of these substances.
“The three additional substances were identified in imported shipments held by Customs at the border. It should not be assumed that new branded products on the market contain one or more of these three new substances. ESR experience is that branded products may contain different substances or different mixtures of substances at different times so that a specific brand is not necessarily made to a consistent “recipe”.
“The methodology used in ESR workplace drug testing is in principle capable of detecting and identifying a range of new substances as they appear and methods are continually being updated. ESR workplace drug testing is currently updating the testing method to include several new synthetic cannabinoid metabolites including two metabolites derived from substances listed in the latest TCDN.
“Unlike the principal Act, under which the “dealing” threshold is applied to the amount of pure drug, the amendment establishing the TCDN regime explicitly refers to the weight of “product” (whatever the concentration of active synthetic cannabinoid-like substance in that product).
“This means that in the absence of other evidence, the TCDN treats someone in possession of less than 56 grams of pure drug the same as someone in possession of less than 56 grams of mixed smoking product, with an obvious discrepancy in the number of potential “doses”.
Reference:
MoDA 1975 4D(4)): Possession by a person of 56 grams or more in total of any products (including cigarettes), or any drug forms (including flakes, tablets, or capsules), each containing some quantity of that temporary class drug is to be treated, for the purposes of this Act, as possession by that person of an amount, level, or quantity at and over which a controlled drug that is specified or described in Part 1 of Schedule 3 is presumed to be for supply.
Dr Leo Schep, Toxicologist, National Poisons Centre, comments:
“The three most recent synthetic cannabinoids to be added to the Temporary Class Drug Notice (JWH-019, JWH-200, AM-1220) are analogues of those already scheduled in August. Presently we have no information on their toxicology whatsoever. As they target the same cannabinoid receptors as the other synthetic analogues, such as JWH-018 and JWH-073, we anticipate users will present at hospitals and medical centres with similar toxicological profiles. These can range from agitation and tachycardia to psychosis, coma and seizures, depending on the duration of exposure and the strength of the analogues present in the product.
“In addition, recent evidence has shown some patients are more susceptible to the risk of psychosis, following their use; we anticipate further instances of patients suffering this condition after using these new analogues.”