Biotech regulators in Britain have started public consultation on experimental reproductive procedures proposed to treat rare mitochondrial diseases, and scientists have been given 5.8 million pounds ($NZ11.1m) to investigate the safety of the techniques.
 And the Nuffield Council on Bioethics is conducting an ethical review on the imnplications of the DNA-swapping technologies, which involve transferring the nuclear DNA of an egg with defective mitochondria to an egg with healthy mitochondria that has been stripped of its nucleus. Tabloid media have portrayed this as producing children with three parents.
And the Nuffield Council on Bioethics is conducting an ethical review on the imnplications of the DNA-swapping technologies, which involve transferring the nuclear DNA of an egg with defective mitochondria to an egg with healthy mitochondria that has been stripped of its nucleus. Tabloid media have portrayed this as producing children with three parents.
The mitochondrion is a cytoplasmic organelle of endosymbiotic origin, responsible for energy production, apoptosis regulation and formation of reactive oxygen species within the cell. It contains a circular DNA with 37 genes with strict maternal inheritance.
Read about mitochondrial diseases in this SMC factsheet
Mitochondrial DNA mutations (mtDNA) passed from a mother to her child have been linked to a number of conditions, such as some forms of muscular dystrophy and type 2 diabetes. As many as 1 in 250 people carry a potentially disease-causing mitochondrial mutation, and recently a number of eye diseases have been shown to be caused by such mutations.
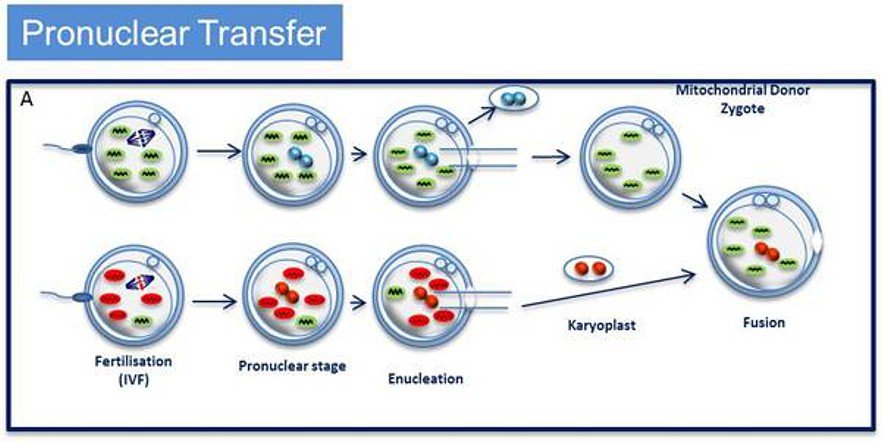
The UK research funding will set up a Wellcome Trust Centre for Mitochondrial Research at Newcastle University, headed by neurologist Professor Doug Turnbull, who will check the safety of two DNA-swapping procedures called pronuclear transfer and maternal spindle transfer. Professor Turnbull has already carried out pronuclear transfer — shifting the nucleus of a fertilised egg into another fertilised egg that lacks mitochondrial mutations and has been stripped of its nucleus — in human eggs that were not suitable for implant and found that 18 out of the 80 resulting embryos developed to the 8-cell stage, and a small number reached the 100-cell blastocyst stage at which implantation normally occurs.
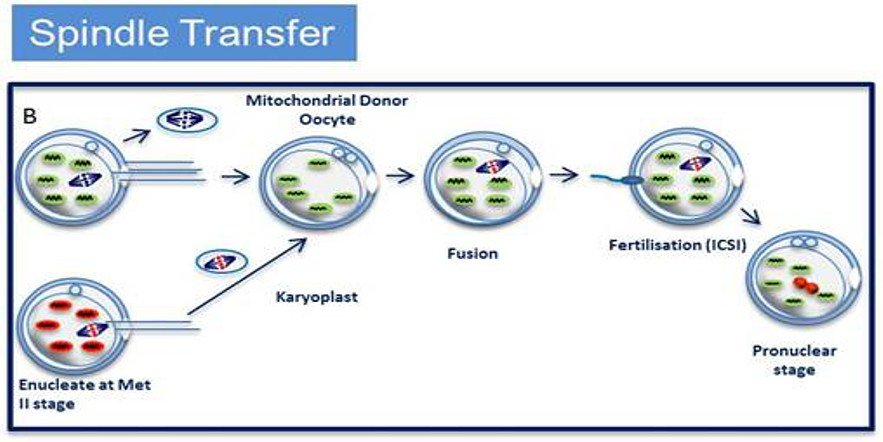
Maternal spindle transfer (MST) involves transferring the chromosomes of an unfertilised egg into another woman’s egg that has been stripped of its nucleus. That hybrid cell is then fertilised in vitro. In 2009, scientists in Oregon reported that two rhesus macaques conceived this way developed normally.
In April last year, Britain’s Human Fertilisation and Embryology Authority (HFEA) called for specific experiments to be done before the technolgy was tried in humans, and the new work will include some of those experiments.
Our colleagues in the UK Science Media Centre collected these responses from scientists:
Professor Sir John Tooke, President of the Academy of Medical Sciences, said
“We warmly welcome news that research to understand, and address the impacts of, mitochondrial disease will continue to be undertaken by scientists in the UK, and that views of the public on this exciting area of research are to be sought.
New techniques to prevent the hereditary transmission of mitochondrial disease could be of clinical benefit in the near future. The views of the public and patients should be taken into account, alongside scientific evidence and expert ethical opinion, when deciding whether new techniques such as these should be permitted. We appreciate the Government’s approach and look forward to hearing the outcomes of this important public dialogue.”
Alastair Kent, Director of Genetic Alliance UK said
“The techniques under development at Newcastle University hold great promise for families affected by conditions caused by faults in mitochondrial DNA, and we are delighted that this work will be able to continue. We hope this next stage of research will bring us closer to being able to offer couples the option to have a child free from these devastating conditions.
“This is a complicated and sensitive area, about which the public must be well informed. We look forward to participating in the Government’s public dialogue, and to highlighting the clear value of a technique which allows couples affected by this problem to have healthy children.”
Professor Robin Lovell Badge, MRC National Institute for Medical Research, said
“Creation of a new research centre and a public consultation will both help to ensure that innovative methods to avoid mitochondrial disease can be developed and, if deemed safe, adopted in a timely fashion. It is important that research on mitochondrial diseases, including the development of innovative methods to avoid these occurring in children, is conducted in a way that recognises the needs and concerns of patients, but also that it has the support of society at large. The new Centre will bring many of the stakeholders together, while the public consultation will be an opportunity to convey the underlying science, including the nature of these often devastating diseases, and to dispel some of the impressions gained from headlines or from those who seem to be against progress in general.
“If the pronuclear and/or spindle transfer methods are adopted clinically, and I shall await with great interest the results of the experiments requested by the HFEA’s scientific review of these methods*, any mitochondrial donor will be crucial to the outcome, namely to the birth of healthy offspring. But I personally do not think that we should get tangled up too much in a debate about whether they constitute a third parent or not. If I had eggs to give, I would be delighted that my mitochondria could help, but they would not convey any character that could be claimed as mine.”
Robert Meadowcroft, Chief Executive of the Muscular Dystrophy Campaign said:
“This research could end the heartbreak of parents passing on these diseases to their children – diseases that can cause devastating symptoms and even death – so it’s imperative that this research is taken forward.
“This technology has been shown to work in the lab so now we must explore it further and move it into clinical trials without delay.
Sharmila Nebhrajani, chief executive of the Association of Medical Research Charities (AMRC) said:
“Right now there are no cures for mitochondrial diseases and no way of helping people avoid passing them on to their children. Research in this area is much needed and AMRC are delighted that the team in Newcastle have funding. If their research is successful it will be vital the health secretary moves quickly to ensure these techniques can be licensed and are available to patients. It is also essential that we all have confidence in the legal and ethical framework around these therapies, and we welcome the Department of Health’s public dialogue as an important step in this process.”
Dr Geoff Watts, Chair of the Nuffield Council on Bioethics inquiry on mitochondrial donation, said:
“The strong interest in mitochondrial disease research shows that it is an important time to be thinking ahead and taking stock of the ethical questions that could arise. With this in mind the Nuffield Council on Bioethics has launched an open call for evidence to seek people’s views on the ethical issues raised by techniques to prevent the transmission of inherited mitochondrial disorders.
“The techniques we are particularly concerned with involve using healthy mitochondria from a donor egg to replace the mother’s unhealthy mitochondria. If these procedures were permitted for treatment, the resulting child is expected to be free from inherited mitochondrial disorders. However, some people may be concerned about the ethical acceptability of these techniques. At this stage of our investigation we are seeking views from a wide range of people on important ethical questions such as ‘Is it acceptable to make changes in the embryo that will then be inherited by future generations?’
UK Science Media Centre Fact Sheet
Mitochondrial DNA
Mitochondrial DNA (mtDNA) is DNA contained in the mitochondria in our cells – these are the energy-generating structures commonly referred to as the ‘batteries’ or ‘powerhouses’ of the cells
Mitochondria have their own genome which is separate from that contained within the cell nucleus – this consists of a circular molecule of DNA, containing the genes necessary for mitochondrial formation – the mitochondria The mitochondrial genome contains around 16,500 base pairs (the nuclear genome contains over 3 billion base pairs) and 37 genes (compared to around 23,000 genes in the nuclear genome).
It is thought to have evolved separately from nuclear DNA, when bacteria containing circular DNA became part of the precursors to cells that exist today – this is shown by the observation of similarities between mitochondrial and bacterial genomes.
Mitochondrial DNA is inherited solely through the maternal side (i.e. from the mother).
Mitochondrial diseases
Mitochondrial diseases are a group of disorders caused by damage to the mitochondrial DNA, or to nuclear DNA that contributes to mitochondrial components. A number of these conditions are associated with mitochondrial myopathy, which feature neuromuscular disease symptoms resulting from failure of the mitochondria to function properly.
Examples of mitochondrial diseases include:
- Neuropathy, Ataxia, and Retinitis Pigmentosa (NARP) – a condition consisting of various symptoms affecting the nervous system, including numbness or pain in the limbs (sensory neuropathy), muscle weakness, and problems with balance and coordination (ataxia) as well as deterioration of light-sensing cells I the retina (retinitis pigmentosa).
- Leigh syndrome – a progressive degenerative disorder affecting the brain and nervous syndrome which mainly appears in infants during their first year of life.
- Myoclonic Epilepsy with Ragged Red Fibres (MERRF) – a rare type of progressive epilepsy characterised by ‘ragged red’ muscle fibres.
- Leber’s hereditary optic atrophy (LHON) — the onset in midlife (average age 30) of painless central visual loss that progresses over a period averaging 4 months, resulting in blindness in both eyes.
They can affect either single or multiple organs, and can appear at any age and with variable frequency. The defects that cause them are transmitted by maternal inheritance, in accordance with the maternal pattern of mtDNA inheritance.
It’s important to note that these are not always clearly distinguishable conditions – there may be various symptoms present than can be attributable to more than one disorder, and careful examination may be required to make a diagnosis.
In most cases, the possibilities of treatment are limited, although there are options for managing the conditions.
Emerging research on mtDNA
Researchers are working on ways to replace damaged mitochondrial DNA in cells as a means of preventing mitochondrial disease being passed on to the next generation. Research in this area is at a stage where treatments could be offered to parents, although it is tightly controlled. It is permitted by the most recent Human Fertilisation and Embryology Act (2008) and is subject to licensing by the Human Fertilisation and Embryology Authority.
The idea is to be able to extract genetic material (nuclear DNA) from the fertilised egg in which the mitochondrial DNA is damaged, and to insert it into another unfertilised egg obtained from a donor in which the mitochondria are undamaged. The DNA is in the form of two pronuclei – one from the egg and one from the sperm that fertilised it – which exist separately before they fuse to form the nucleus with the full set of chromosomes (one set each from mother and father).
The egg is then allowed to develop in vitro with normal mitochondria, as per the (simplified) diagram below.
An alternative emerging technique is maternal spindle transfer, whereby the mother’s nuclear genetic material (the spindle with the chromosomes attached) is transferred from an unfertilised egg into a donor egg which has had its own nuclear material removed; the reconstituted egg is then fertilised with sperm from the patients partner. This is not dissimilar from pronuclear transfer (described above) in that both techniques require donated eggs and involve the transfer of genetic material; but with spindle transfer the extraction of the genetic material takes place prior to fertilisation, whereas pronuclear transfer involves the transfer of the pronuclei after fertilisation has taken place.